Congratulations to vtecmec for winning May/June's Lude Of The Month, with his DIY Turbo BB1 build.
>>> Click Here For Profile <<<

>>> Click Here For Profile <<<

FROST !!!
- mercutio
- LotM Winner
- Posts: 14958
- Joined: Sun Aug 08, 2010 8:45 pm
- My Generation: 5G
- Location: Sunny Manchester
- Has thanked: 1 time
- Been thanked: 4 times
- Contact:
bristol_bb4 wrote:ahhh a 5th gen, i love 5th gens
Dino wrote:I loves the 5th gen really.... just dont quote me on it...
4thgenphil wrote:Mines 4 1/4 unches mate, sorry
http://www.ludegeneration.co.uk/profile ... -t618.html
-
Kristoffer
Like I said, I worded it wrong, and I said simplify it, you have completely lost me, I have no idea about any of that stuffDonald wrote:In what other way can you mean it then?I think you are meaning capillary action then, but that is between materials and liquid, rather than water-water etc.
In simple terms though:
Water deposits onto iron.
Water reacts with atmospheric CO2 to form carbonic acid.
Carbonic acid dissolves iron.
Water begins to separate into hydrogen and oxygen.*
Free oxygen bonds with dissolved iron, electrons are freed, rust happens.*
Electron inbalance = formation of an anode/cathode, electrons flow back to good iron.§
Process is repeated, powered by electron chain (anode-cathode) and electrolyte (water).
** is sort of simultaneous with § (look up electrolysis of water)
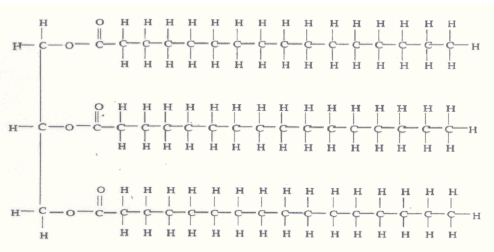
Peanut butter OIL.
Oil structure is generally like this:
The tails with the H density interact with the surface, leave the other end (head) exposed to the water. The tails are hydrophobic so create an area of zero water density, whilst the head end with the O on it is negatively charged, attracting water. It basically makes a barrier.
and you have losted me as well I don't know what that is
So lets try again
- Donald
- Supporter 2015
- Posts: 9894
- Joined: Sun Jun 12, 2011 10:17 pm
- My Generation: 0G
- Location: Earth 3.0
- Been thanked: 7 times
Merc, to be fair I should have probably mentioned iron+water acids too rather than carbonic acids, don't want people thinking all their water is now spontaneously becoming acid  although... it is amphiprotic and Arrhenius/Brønsted-Lowry rules say water acts as it's own acid/base pair but that's probably far too boring.
although... it is amphiprotic and Arrhenius/Brønsted-Lowry rules say water acts as it's own acid/base pair but that's probably far too boring.
Not sure how I can explain it any simpler, Kristoffer I will try draw it out as I can't find a suitable image and words aren't working.
I will try draw it out as I can't find a suitable image and words aren't working.
Not sure how I can explain it any simpler, Kristoffer
-
Kristoffer
No it's cool, you don't have to do that, I just wanted to know what caused rust and what it was made of, but I'll.just read up on it, thanks anyway mateDonald wrote:Merc, to be fair I should have probably mentioned iron+water acids too rather than carbonic acids, don't want people thinking all their water is now spontaneously becoming acidalthough... it is amphiprotic and Arrhenius/Brønsted-Lowry rules say water acts as it's own acid/base pair but that's probably far too boring.
Not sure how I can explain it any simpler, KristofferI will try draw it out as I can't find a suitable image and words aren't working.
No you didn'tandypont wrote:I think you explained it fine Donald, though only the last line was needed.
"It basically makes a barrier"
Glad to have helped with the translation.
- Donald
- Supporter 2015
- Posts: 9894
- Joined: Sun Jun 12, 2011 10:17 pm
- My Generation: 0G
- Location: Earth 3.0
- Been thanked: 7 times
Too late I already did it

You are familiar with how magnets work? As in opposites attract.
Just accept that all atoms are like this, in that they have positive and negative charges relative to each-other. Water is H2O, and H has a slight positive charge, O has a slight negative charge. So in 1. you can see how the opposites attract and form temporary weak 'bonds' (the dashed lines). This is what gives water it's fluidity.
In 2, the same principle applies, except you see in (a) there are all those H's from the last image I posted, all slightly positive charge and because there are so many, the water (even though it has a negative O) can't get in there because of the H+ repelling eachother. At the other end (b), there are O-, but much fewer than H in the tails, so it allows the water to get close enough to bond temporarily (c). This is how the barrier is formed and it's the same for all oils, hence why you see oil slicks in puddles, oil from your pans and water, a buttery knife wont get wet, etc.
-
Kristoffer
You must be bored tonight, if you're helping me or trying to make arse of me I can't make my mind upDonald wrote:that doesn't do it adequately enough for me though Andy! Plus I love this kind of stuff... although if you have any interest in interactions then you should look up leukocyte adhesion, proper Matrix stuff.
Too late I already did itat least the peanut butter repellent. Rust really can't be explained any simpler than water dissolves the metal (but that is missing out a ton of steps).
You are familiar with how magnets work? As in opposites attract.
Just accept that all atoms are sort of like this, in that they have positive and negative charges relative to each-other. Water is H2O, and H has a slight positive charge, O has a slight negative charge. So in 1. you can see how the opposites attract and form temporary weak 'bonds' (the dashed lines). This is what gives water it's fluidity.
In 2, the same principle applies, except you see in (a) there are all those H's from the last image I posted, all slightly positive charge and because there are so many, the water (even though it has a negative O) can't get in there because of the H+ repelling eachother. At the other end (b), there are O-, but only a few, so it allows the water to get close enough to bond temporarily (c). This is how the barrier is formed and it's the same for all oils, hence why you see oil slicks in puddles, oil from your pans and water, a buttery knife wont get wet, etc.
Thanks anyway mate, but I'l just read up
- mercutio
- LotM Winner
- Posts: 14958
- Joined: Sun Aug 08, 2010 8:45 pm
- My Generation: 5G
- Location: Sunny Manchester
- Has thanked: 1 time
- Been thanked: 4 times
- Contact:
oil or an oily substance will sit on the surface of the metal and because oil does not dissolve readily in water it forma a barrier so the water cannot reach the metal to react with it
that is about as simple as it will get
that is about as simple as it will get
bristol_bb4 wrote:ahhh a 5th gen, i love 5th gens
Dino wrote:I loves the 5th gen really.... just dont quote me on it...
4thgenphil wrote:Mines 4 1/4 unches mate, sorry
http://www.ludegeneration.co.uk/profile ... -t618.html
-
Kristoffer
I'm sorry, my apologues, I called it wrong, next you're going to tell me you're into comics and chess and you've been to comic con and you know all batamn and star wars stuff to,Donald wrote:Was genuinely trying to help as I love chemistryit never gets boring.
I still don't get any of that stuff,but thanks for thr help anyway mate,
No, don't get that either thanks anyway